

Chemical substance in vitro/in silico screening system to predict human- and ecotoxicological effects (ChemScreen)

Progress per objective
1. Establish in silico prescreening methods prioritizing in vitro toxicity testing (WP1, leading partner; leading partner)
2. Establish a database and an in silico prescreen to identify potential reproductive toxicants (WP2, FhG)
3. Establishment of sensitive parameters and a medium throughput ‘minimal essential’ in vitro assay panel (WP3, RIVM)
5. Integrative methods to predict in vivo reprotoxicity for both human- and environmental toxicity allowing informed decisions on eventual further testing (WP5, TNO)
6. Integration into one user-friendly tool, including uncertainty assessment (WP6, P&GEN)
7. Efficient dissemination to facilitate widespread implementation (WP7, BDS)
WP1 concentrated on the establishment and selection of in silico prescreening methods to categorize chemicals, mainly using (quantitative) structure-activity relationships (Q)SARs. This was done for major classes of toxicity prioritized in REACH (carcinogenesis, mutagenesis and reproductive (CMR) toxicity and persistent, (very) bioaccumulative toxic (PBT/vPvB) compounds). As a starting point DTU scientists have been using a unique database which comprises abbreviated predictions from more than 70 (Q)SAR models on endpoints for physico-chemical properties, fate, eco-toxicity, absorption, metabolism and toxicity. Positive predictions for carcinogenicity, mutagenicity and reproductive toxicity can be applied to avoid unnecessary animal testing of compounds as replacement for animal tests for these endpoints. As no testing for reproductive effects should be performed in REACH on known genotoxic carcinogens or germ cell mutagens with appropriate risk management measures implemented, as stated in the REACH annexes VIII-X, predictions for genotoxic carcinogens and germ cell mutagens can furthermore be applied to avoid testing for reproductive toxicity. A developmental toxicity screen was carried out as well, but predictivity of this analysis expectedly is rather low. To make the (Q)SAR screening as comprehensive as possible it should be based on as many as possible of the 143,835 chemicals, which were pre-registered (PRS) between 1 June and 1 December 2008. Therefore the work was carried out in collaboration with the Computational Toxicology Group within the Joint Research Centre (JRC), that has generated structure information for 80,413 PRS chemicals, of which 70,983 chemicals were suitable for generation of QSAR predictions for all relevant endpoints Results of the QSAR analysis is depicted in fig 1.
As part of the in vitro pre-screen, it was envisaged to develop an exposure module to identify chemicals that will have no or a negligible systemic availability (and anticipating that gonads and embryos will not be exposed) after exposure via different routes. To that effect, literature searches have been performed to investigate on one hand whether specific chemical properties can be identified to predict internal exposure after exposure via the oral, respiratory and dermal routes, and on the other hand which (publicly available) in silico tools exist leading to reliable prediction of negligible internal exposure. Based on the current knowledge, no cut-offs based on physicochemical properties could be identified for the three main exposure routes below or above which internal exposure is zero or negligible. REACH does provide physicochemical criteria to waive toxicity testing via the respiratory route, but these criteria cannot waive testing via other routes of exposure. Therefore, it was concluded that inclusion of these criteria in a module to predict internal exposure aimed at excluding reproductive toxicity testing based on physicochemical criteria is not a viable option. Instead, the PK models will now be used exclusively in WP5.
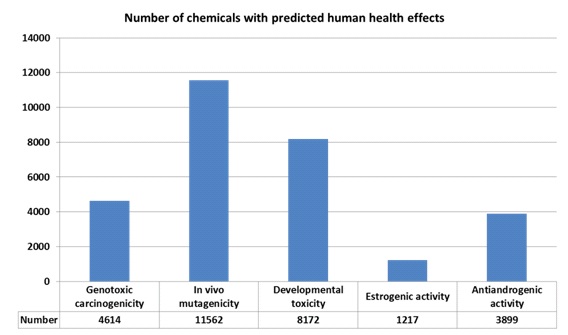
Fig. 1 QSAR analysis using pre-screening tools for human health effects applied to over 70,000 REACH pre-registration chemicals.
WP2 focused on expansion of databases which contain information relevant to reproductive toxicity; RepDose (repeated dose) and FeDTex (fertility and developmental toxicity). First reproductive toxicity studies were identified from peer reviewed publications, as well as other databases provided by other partners. Available studies on reprotoxicity were selected for data entry. In particular, the amount of chemicals in FeDTex will be more than doubled in the course of ChemScreen, mounting up to over 300. A significant overlap with RepDose is created to allow comparison of in vivo data with the same chemical and evaluate possible predictivity of repeated dose toxicity for reproductive toxicity. Meanwhile BDS and Simpple, in collaboration with TNO and other partners are working on a database and software tools in which in vitro screening data can be linked to reference (in vivo) data.
WP2 scientists started a unique collaboration with WP3 and the NCCT on the identification of critical endpoints of reproductive toxicity using combined, in depth analysis of databases present at three different locations (i.e. Fraunhofer Institute, RIVM and NCCT) These results will be extended to ecotox endpoints using the EDUKON database present at the University of Konstanz, which in this period has also been improved and extended substantially (see WP5).
In addition, using database searches RIVM selected two priority areas and relevant substances for defining predictive approaches in reproductive toxicology. These areas were first: sex organ related malformations, allowing ChemScreen expertise in endocrine modulation assays to be employed; and second: neural tube defects, as this is a relatively well-defined and data-rich area of developmental toxicity. These datasets were further refined in collaboration with TNO and FhG and discussed with P&G who will take on the design of predictive tools on the basis of the information available.
The aim of WP3 is the design of a ‘minimal essential’ reproductive toxicity screen, consisting of a number of in vitro assays that are representative of those parameters in reproductive toxicity that are crucial for reproductive hazard assessment. One way to simplify testing schemes is the approach of identifying critical endpoints for reproductive toxicity using literature and database searches, and focusing on development of tests for these endpoints. So far most endpoints identified so far are quite generic and non-specific in nature, which may mean that screening panels should also include quite simple, relatively non-specific tests in addition to more mechanistic assays coming from ChemScreen, as well as through NCCT/TIVSC collaborations.
Meanwhile, progress was obtained at RIVM with the optimization and amendment to higher throughput of two tests that are very likely to be included in the final battery, namely a genomics-based improved embryonal stem cell test, and steroidogenesis assays, in particular four CYP17/19 assays. Two Ph.D students are working on enhancing the predictive value of these assays. In the embryonic stem cell test, transcriptomics readouts are employed to improve the mechanistic readout of the system and improve its predictive value. The CYP17/19 assays are being optimized and microsomes versus whole cell assays are being compared for their predictive value. Furthermore, plans have been made to start working on assays with testicular cell lines in order to fill one of the major gaps in the spectrum of reproductive toxicity testing assays.
WP4 focused on the establishment of high throughput screening methods (ReproScreen HTP) predictive of reprotoxic potential, based on insight in molecular mechanisms that are relevant to reproductive toxicity. These assays comprise a panel of highly specific human CALUX® reporter gene assays at BDS, which has been expanded and now includes more than 25 different cell lines (table 1), that are run in different assay formats (in particular agonistic- and antagonistic mode), totaling about 50 different assays. A systematic approach has been taken developing the screening panel using very sensitive and selective reporter genes, covering a wide range of receptors and signaling pathways that potentially are involved in reproductive toxicity. The approach taken aims to provide several advantages. First of all, by providing a mechanistic base, regulatory acceptance is strongly facilitated. Secondly, this mechanistic base will provide input for decisions on further test requirements and risk assessment. Thirdly, cost, speed, robustness, and quantification is superior in these types of assays. In the fourth place, predictions can be made on both human- and ecotoxicological properties of chemicals, when different prediction models are used. One approach taken to develop additional reporter gene assays for ReproScreen is to use central intracellular pathways that are involved in regulating transcription in a mammalian cell. These pathways include besides nuclear hormone receptors (see above) also reporter gene assays for AP1 (fos/jun complexes, activated via MAPK pathways), NF-kappaB, TCF, nrf-2, p21 and p53 (table 1). Clearly most of these pathways are involved in quite generic responses to toxicants, including ones inducing cytotoxicity, apoptosis and genotoxicity. Since, however, these responses can also be relevant in reproductive toxicity, including teratogenesis, these assays were also selected for the initial panel. In addition, an assays for HIF-1alpha, important for angiogenesis was generated and added to the panel because of its relevance to developmental processes. Also a control cell line, called cytotox CALUX has been generated which constitutively expresses the same luciferase gene under control of the same expression plasmid which is used to generate reporter gene assays for steroid receptors (ie pSG5). This assay has been proved to be a very suitable control for high throughput screening (HTS).
In addition to these highly selective CALUX HTS reporter gene assays, assays are being established in more complex, differentiated cells such as murine ES cells by introducing reporter systems for signalling pathways controlling key differentiation pathways in embryonic development (so-called ReproGlo assays). In this way, assays for up to 5 signalling pathways that are important for early embryonic development (Wnt/, SHH-, TGFβ-, Delta/Notch-, and the RTK-signalling pathway’) aim to be established. This part of the work experienced some delays so far due to problems with the stability of selected clones. The results of the currently running feasibility studies (see below) and previous studies are at the moment supplemented with the testing of additional chemicals of interest, and this data will then be analyzed in order to define an applicability domain for the ReProGlo (Wnt-D3) assay.
All CALUX and ReProGlo assays have now been automated in 384 or 96 wells format, respectively, and a series of reference compounds has been used to deselect less informative assays and identify missing endpoints. A further (de)selection of the assay panel is being made based on the critical endpoints identified in WP3, and additional chemical testing. Data storage and analysis is being set up in collaboration with WP6 partners.
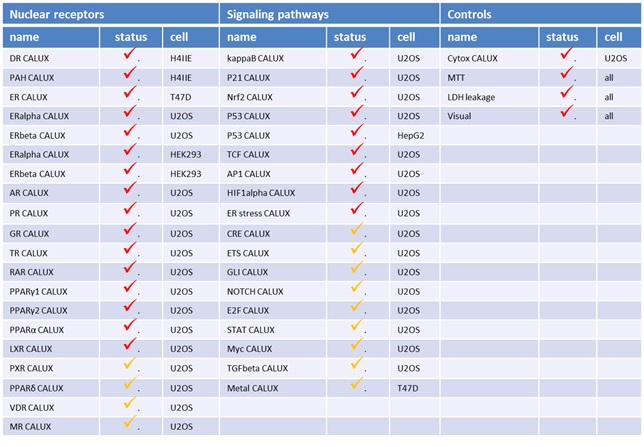
Table 1.Currently available stable CALUX assays. Checked in red: stable. Orange: transient
WP5 is establishing integrative methods to predict in vivo reprotoxicity for both human- and environmental toxicity using in vitro benchmark (threshold) concentrations as a starting point. Prediction of the correct in vivo dose level at which adverse effects can be expected is one of the key issues. High throughput pharmacokinetic models are being set up describing the bioavailability via relevant routes (oral, dermal, inhalation) and the pharmacokinetics of the most relevant reference chemical classes.
A PBPK modelling strategy has been developed to translate in vitro concentrations to in vivo dose levels. Instead of building custom models for each chemical (class) as planned, it was chosen to develop one generic PBPK model framework describing all relevant compartments, physiological processes and exposure routes. The generic model with its (chemical-independent) anatomical and physiological parameter values in principle applies to all chemicals. The PK of specific compounds has been addressed by providing chemical-specific parameters (relating to permeability, partitioning and clearance) that can be obtained by either in silico predictions or routine in vitro measurements, as input to the generic model. To this effect, an algorithm has been implemented to predict the binding and partitioning behaviour of compounds in water, protein and lipid phases of blood (plasma and red blood cells) and tissues (interstitial fluid and cells). This algorithm takes as input readily obtainable chemical properties. It can also be used to predict binding and partitioning behaviour in culture medium if its pH and protein and lipid fractions are known, and thus to correct for differences in unbound concentrations between in vitro and in vivo. This generic framework is more compatible with the rapid screening character of the ChemScreen tool as a whole, and allows PK prediction of a much larger number of compounds in a relatively short time. Till now, the collection of chemical-specific parameter values and the comparison of model simulations with in vivo PK data has been performed for the first list of test compounds. In addition, in vitro-in vivo extrapolation of the available data so far, determined by other partners of the ChemScreen project, has been performed.
In risk assessment, methods determining defined conserved mechanisms of toxicity have a great advantage over more classical black-box approaches, such as morphological endpoints. This is because interpretation of data is much more straightforward. By focusing on molecular mechanisms conserved in various species (e.g. various receptor-mediated processes) possibilities are explored to predict not only for humans but also for aquatic organisms (particularly fish) and thus for ecotoxicological effects. For this extrapolation methods are being designed to establish if there are conserved molecular mechanisms in aquatic organisms responsive to the action of different chemicals. As one of the first steps an expansion and restructuring of the large EDUKON ecotox database (at UKON) has been carried out as well as an inventory of possible chemo-biological interactions that can be related to distinct molecular mechanisms.
WP6 is devoted towards the setup of a software tool that integrates the methods, modules and databases generated in WPs 1-5. It aims to be a flexible and open tool that can be adapted and used for applications beyond the scope of REACH and in the post-REACH period. The tool will take into account the existing landscape of related IT tools (IUCLID5, OECD Toolbox, OSIRIS Web tool, etc.), and will interface with them as required. Concept schemes and the basic design of the software tool have been established at SIM and PGEN. Data integration and analysis methods are being developed using a Bayesian Network (BN) approach using data from various sources including that of the first ChemScreen HTP screens and data from NCCT collaborations. This work will increase in intensity one the full ChemScreen dataset is available.
ChemScreen focuses on delivering a practical approach how to use molecular screening tools for reproductive toxicants in the context of chemical risk assessment in REACH and related programs.
For the dissemination of the project’s results (WP7) various channels are used, including publications, folders, news items (see project’s website: www.chemscreen.eu), participation to and organisation of meetings with scientists and various stakeholders, etc. The approach includes integrated testing strategies, molecular screening tools combined with more apical tests and various in silico methods, including QSAR. The project aims to collaboratively generate an efficient innovative testing strategy combining unique expertise of the participants. To attain this level of interaction, frequent meetings, and workshop have been organized with the partners and the Scientific Advisory Board. In 2011/2012 two general project meetings were organized and three special workshops. These included workshops on topics that are relevant to the scientific program, including one on integrated testing strategies and in silico methods and two on the organization of various feasibility studies. Beginning 2012 an open symposium was organized aimed at stakeholder interaction. At this meeting results of ChemScreen, ToxCast and related molecular screening programs were presented and the possibilities to use such data in chemical risk assessment were discussed.
As one of the project’s first deliverables ChemScreen’s strategy has been further defined and published in the form of a position paper (Van der Burg et al 2011). In addition, ample attention is paid to validation and regulatory acceptance of the test methods developed in ChemScreen. To this end close contacts with relevant ECVAM and OECD working groups, scientists and regulators are being established. Novel science- and performance criteria-based methods will be employed to validate the ReproScreen system, and allow rapid acceptance. Methods aim to be robust and simple allowing widespread dissemination using ample expertise available at BDS to disseminate bioassays worldwide. Methods to screen for endocrine disrupting compounds, such as the ER- and AR CALUX assay already were prevalidated in the context of the ReProTect FP6 program, and subsequently have been submitted to ECVAM to allow formal validation. Since our battery of tests of reporter gene assays is very comparable to these tests, they may be used as “validation anchors” in a screening battery. Through close collaboration, synergy will be attained with ongoing framework projects, and the NCCT molecular screening programs. In order to prevalidate our screening battery and select relevant tests a feasibility study has been initiated early in the project using a set of reproductive toxicants that are run in all available tests.
As an important integrative effort to test the feasibility of using the ChemScreen approach to screen for reproductive toxicants, a ring trail has been initiated. RIVM has been involved in the selection of the first set of chemicals for the ChemScreen ring trial, based on available information about the toxicity profiles of chemicals. A selection of twelve chemicals was made with varying toxicity profiles including reproductive and developmental toxicants as well as endocrine active substances. The outcome of the ring trial, after data collection by BDS, is now being presented in a manuscript in preparation. In addition, a follow-up ring trial is being discussed among ChemScreen partners as well as with the related US STAR project partners. At TNO Chemicals have been selected to be tested in the second feasibility study. The selection was designed to create small groups of structurally similar chemicals with similar and dissimilar reprotoxic properties in order to develop and evaluate read-across approaches.
1. Van der Burg, B., Kroese, E.D., Piersma, A.H. (2011) Towards a Pragmatic Alternative Testing Strategy for the Detection of Reproductive Toxicants. Reproductive Toxicology 31, 558-561.
2. Sonneveld, E., Pieterse, B., Schoonen, W., Van der Burg, B. (2011) Validation of in vitro screening models for progestagenic activities: inter-assay comparison and correlation with in vivo activity in rabbits. Toxicology in Vitro. 25: 545-554.
3. Gijsbers, L., Man, H.Y., Kloet, S.K., De Haan, L.H.J., Keijer, J., Rietjens, I.M., Van der Burg, B., Aarts, J.M. (2011) Stable reporter cell lines for PPARγ-mediated modulation of gene expression. Analytical Biochemistry 414:77-83.
4. Suzuki G, Tue NM, van der Linden S, Brouwer A, van der Burg B, Lamoree M, van Velzen M, Someya M, Takahashi S, Isobe T, Tajima Y, Yamada T, Takigami H, Tanabe S (2011) Identification of major dioxin-like compounds and androgen receptor antagonist in Acid-treated tissue extracts of high trophic-level animals. Env Science & Technol., 45:10203-11
5. Gauggel S., Derreza-Greven C., Wimmer J., Wingfield M., van der Burg, B., and Dietrich D.R. (2012) Characterization of biologically available wood combustion particles in cell culture medium. ATLA, in press.
6. Van der Burg, B., Van der Linden, S.C., Man, H.Y., Winter, R., Jonker, L., Lussenberg, B., Brouwer, A. A panel of quantitative CALUX® reporter gene assays for reliable high throughput toxicity screening of chemicals and complex mixtures. In "High throughput screening methods in toxicity testing" (P. Steinberg, ed). John Wiley and Sons, Inc. New York, in press.
7. Schulpen S. H.W, J.F. Robinson, J.L.A. Pennings, D.A.M. van Dartel, A H. Piersma. Dose response analysis of monophthalates in the murine embryonic stem cell test assessed by cardiomyocyte differentiation and gene expression, in press.
8. Schulpen SWJ, Aldert H. Piersma The Embryonic Stem Cell Test In: Teratogenicity Testing Methods and Protocols; Ed. Paul Barrow, Humana Press, in press.
