Chemical substance in vitro/in silico screening system to predict human- and ecotoxicological effects (ChemScreen)

Background
Registration Evaluation Authorisation of
Chemicals (REACH) The current system of risk assessment of chemicals
is complex, very resource-intensive and has been working extremely
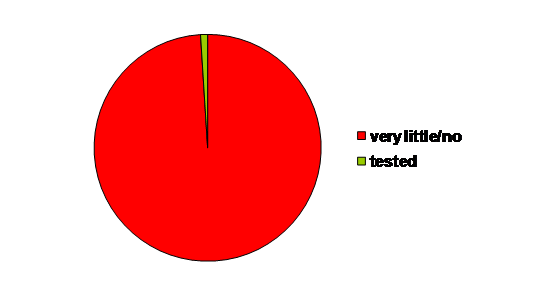
slowly. In 1981, of the 100,106 chemicals on the market, only
1% was tested on hazardous properties, a situation which has not
changed substantially since then (see EU White Paper: Strategy for
a future Chemicals Policy, 2001). Because of this a major regulatory
endeavor has been initiated, the REACH legislation aiming to greatly
speed up this process. However, when traditional animal tests are
used progress of REACH will be seriously hampered by:
Ethics:
resistance to the excessive use of animals.
Costs:
particular those linked to labor intensive animal testing
Capacity:
lack of capacity to carry out these tests.
Speed:
the use of the same traditional methods will not allow major advances
in speed of the process to be made.
These obstacles necessitate the serious
consideration of alternative, non-animal (in silico and in vitro)
testing strategies.
Priority areas for reduction and replacement
of animal testing
Reproductive toxicity testing alone will
be the major consumer of animals (more than 60% of all). Therefore,
it is the prime area within REACH in which alternative methods
can lead to a vast reduction of animal experimentation. ChemScreen
will focus on alternative methods to test for reproductive toxicity.
To complete the screening
system the current program will concentrate on the following
tasks:
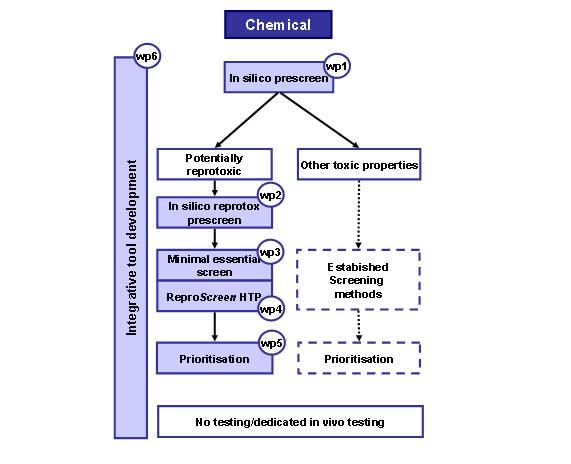
- Establish in silico prescreening and toxicity prediction methods prioritizing in vitro toxicity testing (WP1, leading partner; DTU)
- Establish a database and an in silico prescreen to identify potential reproductive toxicants (WP2, FhG)
- Establishment of sensitive parameters and a medium throughput ‘minimal essential’ in vitro assay panel (WP3, RIVM)
- Establish a high throughput mechanistic pathway screen, ReproScreen HTP, for reproductive toxicants (WP4, EKUT)
- Integrative methods to predict in vivo reprotoxicity allowing informed decisions on prioritization for eventual further testing (WP5, TNO)
- Integration into one user-friendly tool (WP6, P&GEN)